- Home
- »
- Basics
Basics
REACH
cPreparation of REACH Mini Registration Dossiers, that is quick, professional registration at a minimal cost!
REACH registration is much more complex than we first thought. Without the help of a good consultant, or participating at REACH conferences on a regular basis it is a brave decision to complete a mini registration dossier.
If you watch this presentation below you will get an answer to your basic questions related to the REACH registration.
If you still have questions, please read the REACH part in this homepage or call me.

If you choose to have my company as your consultant to prepare your mini registration dossier, we will ensure 100% money back guarantee that your registration dossier will successfully pass first. This means we will return your money if the submission does not pass for first trial.
If your buyer produces from your chemical substance another chemical substance, then you have a good chance for the cheaper intermediate registration. According to REACH the intermediate does not need to remain within the premises in all cases, it is quite possible that your end product is an intermediate according to REACH.
A substance is an intermediate if all following conditions are met:
- The substance is manufactured to be itself converted into another substance on an industrial site,
- The outcome of the chemical processing is another manufactured substance on its own but not another substance in an article.
The intermediate registration is significantly cheaper than a normal chemical substance registration.
If you watch this presentation below you will get an answer to your basic questions related to the REACH intermediate registration.
If you still have questions, please read the REACH part (Intermediate manufacturers) in this homepage or call me.

If you choose to have my company as your consultant to prepare your intermediate lead or mini registration dossier, we will ensure 100% money back guarantee that your registration dossier will successfully pass first. This means we will return your money if the submission does not pass for first trial.
Preparation of a REACH lead registration dossier
Don’t wait until another company will be the lead registrant in 2018, control the price and be you the lead registrant! It will save you money.
If you watch this presentation below you will get an answer to your basic questions related to the REACH lead registration dossier.
If you still have questions, please read the REACH part in this homepage or call me.

If you choose to have my company as your consultant to prepare your lead registration dossier, we will ensure 100% money back guarantee that your registration dossier will successfully pass first. This means we will return your money if the submission does not pass for first trial.
Hunting for a lead registrant
Advise from an experienced REACH consultant: Find out the LoA price from the lead registrant, if no lead registrant yet, be you in order to control the price. I will tell you how to find out the lead registrant in this presentation below.
REACH training basic, intermediate, advanced
Advise from an experienced REACH consultant: If you are a new chemical responsible at your company, you will be more confident in your job if you take a REACH and GHS training. Do not risk the compliance of your company, come to a REACH training or ask for a REACH audit.
If you watch this presentation below you will get an answer to your basic questions related to the REACH training.
If you still have questions, please read the REACH part in this homepage or call me.
CLP
CLP is an EU regulation, which falls into the “strongest” compulsory legal regulation category. This means that this regulation applies in uniform to all member countries, without the need to adjust to the local, national laws, and it must be translated in the same way, word by word, in each EU member country.
The Regulation (EC) No 1272/2008 on classification, labelling and packaging of substances and mixtures (CLP Regulation or simply “CLP”) which entered into force on 20 January 2009 in the EU countries and is expected to enter into force in Norway, Iceland and Liechtenstein as well in the near future. CLP will supersede DSD (67/548/EGK) and DPD (1999/45/EC) over time. These directives will finally be repealed after a transitional period, i.e. on 1 June 2015.
How can GHS-expert Ltd. help you with it’s services?
- Easily accessible, professional CLP (GHS) classification, labeling and notification, using the GHS-expert software – for those who choose to do the classification themselves.
- Full scale classification and labeling with the help of GHS Expert Ltd. Company
- Notification of the classification with the help of GHS-expert Ltd.
In the framework of the United Nations Organization (UNO) the harmonized criteria of the classification and labeling, as well as the general principles of their application, were carefully developed over a 12 year period. As a result, the “global harmonized system of chemical substance classification and labeling”, the GHS ( Global Harmonization System) was formed.
The CLP regulation is none other, than the integration of the internationally accepted GHS criteria into the EU community laws.
Outside of the European Union, there are more and more countries accepting the GHS criteria in their legal system.

(purple: implemented | green: implementation in process | yellow: negotiation started)
The objective of the CLP regulation
- One of the main aims of CLP is to determine whether a substance or mixture displays properties that lead to a classification as hazardous.The classification criteria refer to substances and mixtures placed on the market, articles with explosive or pyrotechnic effect, independent of the quantity of the given substance manifactured, imported and put on the market. The classification requirement also refers to manufactured or imported quantities under 1t/year.Substances not in the market must also be classified, but only those for which the REACH regulation requires registration and notification, eg. monomers, on-site isolated intermediates, transported isolated intermediates, PPORD substances.
- Harmonizing the labeling of hazardous substances and mixtures. Labeling and packaging requirements refer to hazardous substances and mixtures.
- Harmonizing the packaging of hazardous substances
See them in CLP Article 1 (2)
- radioactive substances and mixtures
- substances and mixtures which are subject to customs supervision
- non-isolated intermediates;
- substances and mixtures for scientific research and development, which are not placed on the market
Waste - in the interests of defence.
- medicinal products, veterinary medicinal products
- cosmetic products
- medical devices
- food or feeding stuffs
A manufacturer, importer, downstream user (including formulator) or distributor (including retailer) should label any substance or mixture requiring labelling and contained in packaging, see above, before you place it on the market (CLP Article 4(4)). This applies also to producers and importers of articles which are explosive according to the criteria in Part 2 of Annex I to CLP.
A distributor does not need to classify from scratch for the purposes of labelling, but may take over the classification of a substance or mixture from your supplier, provided it is derived in accordance with CLP Title II (CLP Article 4(5), CLP Articles 5-16).
Your labels should be written in the official language(s) of the Member State(s) where the substance or mixture is placed on the market, unless the Member State(s) concerned provide(s) otherwise. In this connection you may wish to check the relevant national legislation where such provisions are laid down.
In general, you can use more languages than those required by the Member States provided that the same information appears in all languages used (CLP Article 17(2)) and that the label still fulfils the requirement of being easy to read (CLP Article 31).
Labelling elements
- The name, address and telephone number of the supplier/s of the substance or mixture
- The nominal quantity of the substance or mixture in the packages made available to the general public, unless this quantity is specified elsewhere on the package
- Product identifiers and where applicable
- Hazard pictograms
- Signal word
- Hazard statements
- Appropriate precautionary statements and
- Supplemental information

Label and pictogram sizes
| Capacity of the package | Dimensions of label /mm/ | Dimensions of pictograms /mm/ |
| < 3 litres | If possible at least 52 x 74 | At least 16 x 16 |
| > 3 litres but < 50 litres | At least 74 x 105 | At least 23 x 23 |
| > 50 litres but < 500 litres | At least 105 x 148 | At least 32 x 32 |
| > 500 litres | At least 148 x 210 | At least 45×45 |
Introductory Guidance on the CLP Regulation
CLP Regulation (EC) No 1272/2008 on classification, labelling and packaging (CLP) of substances and mixtures
If you have further questions please write me an e-mail: agnes.botos@ghsexpert.com
The CLP Regulation entered into force on 20 January 2009. However, not all the provisions of the CLP Regulation will be obligatory immediately: There are the transitional provisions set out in CLP Article 61 which define two target dates that affect the classification, hazard communication and packaging of hazardous substances and mixtures, namely 1 December 2010 and 1 June 2015.
The table below helps you understand the deadlines of the regulation. Because it is very important to understand these deadlines, I have put onto my web site the corresponding sections of the instructions.
| Time | Chemical material | Classification** | Notification | Safeti data sheet*** | Labelling and packing* |
| 2009.01.20 – 2010.11.30 | Substance | DSD | DSD or CLP | DSD | DSD |
| or DSD & CLP | CLP | DSD & CLP | CLP | ||
| Mixture | DSD | – | DSD | DSD | |
| or DSD & CLP | – | DSD & CLP | CLP | ||
| 2010.12.01 – 2015.05.31 | Substance | DSD & CLP | CLP | DSD & CLP | CLP |
| Mixture | DSD | – | DSD | DSD | |
| or DSD & CLP | – | DSD & CLP | CLP | ||
| from 2015.06.01 | Substance | CLP | CLP | CLP | CLP |
| Mixture | CLP | – | CLP | – |
* substances classified, labelled and packaged in accordance with Directive 67/548/EEC and already placed on the market before 1 December 2010, are not required to be relabelled and repackaged in accordance with this Regulation until 1 December 2012.
** mixtures classified, labelled and packaged in accordance with Directive 1999/45/EC and already placed on the market before 1 June 2015 are not required to be relabelled and repackaged in accordance with this Regulation until 1 June 2017.
*** Safety data sheets should be issued for hazardous substances and mixtures.
From 20 January 2009 the following rules apply
- until 1 December 2010, substances must continue to be classified, labelled and packaged in accordance with DSD. However, a substance may also be classified, labelled and packaged according to CLP before this date. When this is done, the labelling and packaging provisions of DSD shall no longer apply to the substance. This means that labelling and packaging must respect the provisions of CLP.
- until 1 June 2015, mixtures must continue to be classified, labelled and packaged in accordance with DPD. However, a mixture may also be classified, labelled and packaged according to CLP before this date. When this is done, the labelling and packaging provisions of DPD shall no longer apply to the mixture. This means that labelling and packaging must respect the provisions of CLP.
- until 1 June 2015, the classification of a substance according to DSD must be provided in the Safety Data Sheet. This will both apply to Safety Data Sheets for substances on their own and to Safety Data Sheets for mixtures containing these substances
- until 1 December 2010, if a substance is classified, labelled and packaged according to CLP, the CLP classification must appear on the Safety Data Sheet, alongside the classification based on the DSD. However, a supplier may choose to identify the CLP classification of a substance in advance of applying CLP to it in full. Where this happens, the supplier may include this information on the accompanying Safety Data Sheet, under the ‘other information’ heading
- until 1 June 2015, the classification of a mixture according to DPD must be provided in the Safety Data Sheet.
- until 1 June 2015, if a mixture is classified, labelled and packaged according to CLP, the CLP classification must appear on the Safety Data Sheet, alongside the classification based on the DPD. However, a supplier may choose to identify the CLP classification of a mixture in advance of applying CLP to it in full. Where this happens, the supplier may include this information on the accompanying Safety Data Sheet, under the ‘other information’ heading.
- from 20 January 2009 Title V starts to apply, so manufacturers, importers and downstream users can submit proposals for harmonised classification to the Agency (CLP Article 37(2)) and shall submit a proposal to a Member State Competent Authority in case they have new information which may lead to change in harmonised classification and labelling (CLP Article 37(6), see also section 22 of the guidance document).
From 1 December 2010 the following rules shall apply
- substances must be classified in accordance with both DSD and CLP;
- substances must be labelled and packaged in accordance with CLP only, but substances already classified, labelled and packaged according to DSD and placed on the market (i.e. “on the shelves”) before 1 December 2010 will only have to be re-labelled and re-packaged by 1 December 2012;
- until 1 June 2015, mixtures must continue to be classified, labelled and packaged in accordance with DPD. However, a mixture may also be classified, labelled and packaged according to CLP before this date. When this is done, the labelling and packaging provisions of DPD shall no longer apply to the mixture. This means that labelling and packaging must respect the provisions of CLP;
- until 1 June 2015, the classification of a substance according to DSD must be provided in the Safety Data Sheet, in addition to the CLP classification. This will both apply to Safety Data Sheets for substances on their own and to Safety Data Sheets for mixtures containing these substances;
- until 1 June 2015, the classification of a mixture according to DPD must be provided in the Safety Data Sheet;
- until 1 June 2015, if a mixture is classified, labelled and packaged according to CLP, the CLP classification must appear on the Safety Data Sheet, alongside the classification based on the DPD. However, a supplier may choose to identify the CLP classification of a mixture in advance of applying CLP to it in full. Where this happens, the supplier may include this information on the accompanying Safety Data Sheet, under the ‘other information’ heading.
From 1 June 2015 the following rules shall apply
- substances must be classified in accordance with CLP only
- mixtures must be classified, labelled and packaged in accordance with CLP only, but mixtures already classified, labelled and packaged according to DPD and placed on the market (i.e. “on the shelves”) before 1 June 2015 will only have to be re-labelled and re-packaged by 1 June 2017; and
- substance and mixture classifications according to CLP must be provided in the Safety Data Sheet.
The classification criteria refer to substances and mixtures placed on the market, articles with explosive or pyrotechnic effect, independent of the quantity of the given substance manifactured, imported and put on the market. The classification requirement also refers to manufactured or imported quantities under 1t/year.
Substances not in the market must also be classified, but only those for which the REACH regulation requires registration and notification, eg. monomers, on-site isolated intermediates, transported isolated intermediates, PPORD substances.
CLP includes provisions for two sorts of classification:
- self-classification
- harmonised classification.
The harmonized classification of substances (several thousands of substances) is included in the table 3.1 of appendix VI. of the CLP regulation. It is not possible to diverge from the harmonized classification, manufacturers and importers are obligated to use this classification. Authorities of the membering countries, as well as the industry may suggest new substances for the Harmonized Classification listing. As a result, the table 3.1 in Annex VI. of the CLP will be refreshed in the future, as long as the Committe decides in favor of further harmonized classifications.
You must also decide, in connection with the classification, to what extent you wish to use the Translation tables provided in Annex VII. of the CLP, which match the old classifications ( R phrases) to the CLP classifications. Direct matching in most cases is not possible from the Translation tables.
If a chemical substance is not included in the Harmonized Classification list, then it must be classified according to the Tranlsation table of Annex VII. and the classification and labeling requirements of Annex I.
You should consider whether you have the expertise to make judgements about the adequacy and validity of the hazard information obtained. If not, you may need to consult an expert. You, or the expert involved, should examine the information you have gathered to ascertain whether it is adequate and reliable for the purpose of classification.
The information should relate to the forms or physical states in which the substance is used or placed on the market and in which it can reasonably be expected to be used.
If the information available to you is not sufficient to conclude on the physical hazards of your substance, then you must perform new tests to determine the physical hazards if required in Part 2 of Annex I to CLP. For the determination of the health and environmental hazards of your substance, you may decide to perform new testing provided you have exhausted all other means of generating information.
Classifying mixtures
The classification of mixtures under CLP is for the same hazards as for substances. As a general rule and as with substances, available data on the mixture as a whole should primarily be used to determine the classification. If this cannot be done, further approaches to mixture classification may be applied the so-called bridging principles for some health and environmental hazards, using data on similar tested mixtures and information on individual hazardous ingredient substances.
Physical hazard
Explosive
- Unst. Expl.
- Expl. 1.1
- Expl. 1.2
- Expl. 1.3
- Expl. 1.4
- Expl. 1.5
- Expl. 1.6
Flammable gas
- Flam. Gas 1
- Flam. Gas 2
Flammable aerosol
- Flam. Aerosol 1
- Flam. Aerosol 2
Oxidising gas
- Ox. Gaz 1
Gases under pressure
- Press. Gas. (*)
Flammable liquid
- Flam. Liq. 1
- Flam. Liq. 2
- Flam. Liq. 3
Flammable solid
- Flam. Sol. 1
- Flam. Sol. 2
Self-reactive substance or mixture
- Self-react. A
- Self-react. B
- Self-react. CD
- Self-react. EF
- Self-react. G
Pyrophoric liquid
- Pyr. Liq. 1
Pyrophoric solid
- Pyr. Sol. 1
Self-heating substance or mixture
- Self-heat 1
- Self-heat 2
Substance or mixure which in contact with water emits flammable gas
- Water-react. 1
- Water-react. 2
- Water-react. 3
Oxidising liquid
- Ox. Liq. 1
- Ox. Liq. 2
- Ox. Liq. 3
Oxidising solid
- Ox. Sol. 1
- Ox. Sol. 2
- Ox. Sol. 3
Organic peroxide
- Org. Perox. A
- Org. Perox. B
- Org. Perox. CD
- Org. Perox. EF
- Org. Perox. G
Substance or mixture corrosive to metals
- Met Corr 1
Health hazard
Acute toxicity
- Acute Tox. 1
- Acute Tox. 2
- Acute Tox. 3
- Acute Tox. 4
Scin corrosion/irritation
- Skin Corr. 1A
- Skin Corr. 1B
- Skin Corr. 1C
- Skin Irrit. 2
Serious eye damage/eye irritation
- Eye Dam. 1
- Eye Irrit. 2
Respiratory/skin sensitization
- Resp. Sens. 1
- Skin Sens.. 1
Germ cell mutagenicity
- Muta. 1A
- Muta. 1B
- Muta. 2
Carcinogenicity
- Carc. 1A
- Carc. 1B
- Carc. 2
Reproductive toxicity
- Repr. 1A
- Repr. 1B
- Repr. 2
- Lact.
Specific target organ toxicity – single exposure
- STOT SE 1
- STOT SE 2
- STOT SE 3
Specific target organ toxicity – repeated exposure
- STOT RE 1
- STOT RE 2
Aspiration hazard
- Asp. Tox. 1
Environment hazard
Hazardous to the aquatic environment
- Aquatic Acute 1
- Aquatic Chronic 1
- Aquatic Chronic 2
- Aquatic Chronic 3
- Aquatic Chronic 4
Hazardous for the ozone layer
- Ozone
As a manufacturer of substances, an importer of substances or mixtures or as downstream user, you should assemble and keep available all the information that you used for the classification and labelling of your substance or mixture. This information should be kept for at least 10 years after you last supplied this substance or mixture (CLP Article 49).
The Competent Authority/ies or the enforcement authority of the Member State where you are established or the Agency may request all the information you used for the purpose of classification and labelling under CLP.
Example:
Classification of disodium metasilicate EC#: 229-912-9
CLP annex VI. 3.1 table: List of harmonised classification
| CLP notification by legal entity | |||
| Classification | Labelling | ||
| Hazard Class and Category Code(s) | Hazard statement Code(s) | Pictogram, Signal Word Code(s) | Hazard statement Code(s) |
| Skin Corr. 1 B | H314 | GHS05 | H314 |
| STOT SE 3 | H335 | GHS0 | H335 |
| Dgr | |||
Labelling table
| Label elements for skin corrosion /Skin Corr. 1 B/ | Specific target organ toxicity after single exposure /STOT SE 3/ | ||
| Classification | Category 1B | ||
| GHS Pictograms | 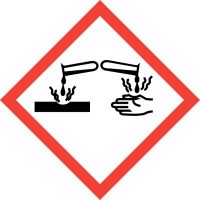 |
 |
|
| Signal Word | Danger | Warning | |
| R-PHRASES | Hazard Statement | H314: Causes severe skin burns and eye damage | H335: May cause respiratory irritation |
| S-PHRASES | Precautionary Statement Prevention |
P260 | P261 |
| P264 | P271 | ||
| P280 | |||
| Precautionary Statement Response |
P301+P330+P331 | P304+P340 | |
| P303+P361+P353 | P312 | ||
| P363 | |||
| P304+P340 | |||
| P310 | |||
| P321 | |||
| P305+P351+P338 | |||
| Precautionary Statement Storage |
P405 | P403+P233 | |
| P405 | |||
| Precautionary Statement Disposal | P501 | P50 |
A manufacturer or importer (or a member of a group of manufacturers or importers) who places a substance on the market will have to notify certain information to the Agency (CLP Article 40) if your substance is:
- subject to registration under REACH (? 1 tonne/year) and placed on the market (CLP Article 39(a)). Please note that a substance that you have already registered under REACH shall not be notified further by you in case the information to be notified has already been provided as part of the registration dossier. This will also apply to certain substances contained in articles where REACH Article 7 provides for their registration;
- classified as hazardous under CLP and is placed on the market, irrespective of the tonnage (CLP Article 39(b)); or
- classified as hazardous under CLP and is present in a mixture above the concentration limits specified in Annex I of CLP or as specified in Directive 1999/45/EC, which results in the classification of the mixture as hazardous, and the mixture is placed on the market (CLP Article 39(b)).
If you are a downstream user who formulates a mixture, a distributor or a producer of articles in the meaning of REACH Article 7, you do not need to notify to the Agency. This is because the notification for your substance will have occurred already at an earlier stage in the supply chain.
For substances which are placed on the market before and still, or again, on 1 December 2010, the deadline for notification to the inventory is one month after this date, i.e. 1 January 2011. However, as 1 January is a Saturday and 2 January falls on a Sunday, the notification deadline is in practice the 3 January 2011. The same applies for substances that are placed on the market on 1 December 2010 for the first time.
Prospective notifiers should bear in mind that the period from 24 December 2010 to 2 January 2011 will be an official holiday for ECHA. Accordingly, it is recommended that, where possible, a notification is submitted before 24 December 2010, as this would allow for any technical problems with the submission tool to be resolved in a timely manner, thus reducing the risks of difficulties in making a successful notification.
For example, you as manufacturer or importer supply a substance on 8 November 2010, then you stop supplying for a while, and then you again supply it on 1 February 2011. The obligatory one month notification deadline is to be calculated from 1 February 2011 and therefore your notification is due on 1 March at the latest. You can, of course, voluntarily notify before 1 December 2010 already.
If you register your substance according to REACH before December 1, 2010, then your lead registrant will complete the CLP (GHS) notification for your company, along with the sending of the registration dossier. In this case your company does not need to do a separate CLP (GHS) notification.
If you do not register your substance according to REACH prior to December 1, 2010, then you must notify it by January 1, 2011.
You must also notify chemical substances included in the Harmonized Classification List in table 3.1 in Annex VI. of the CLP.
Summary table of notfication:
| CLP notification by legal entity | ||||||
| Substance | Notification deadlinie | Notifier | Not notifier | Tonnage | Hazardous criterium | Placing on the market |
| REACH registration in 2010 | 2010. dec. 1. | Lead registrant | Registrant of 2010 | Above 1 tonnes | hazardous and non-hazardous | yes |
| REACH registration in 2013 & 2018 and placed on the market | 2010. dec. 24. | Registrant of 2013 & 2018 | Only-representative since the importer will do it | Above 1 tonnes |
hazardous and non-hazardous | yes |
| REACH registration in 2013 & 2018 and not placed on the market on 1st of December 2010 | optional on 2010. dec. 24 , and obligatory after 1 month of placed on the marke | Registrant of 2013 & 2018 | Only-representative since the importer will do it | Above 1 tonnes | hazardous and non-hazardous | yes |
| Classified as hazardous and placed on the market after 2010. dec 1 if it was not notified with REACH registration (import means placing on the market | obligatory after 1 month of placed on the market | manufacturer, importer | – | Any quantity | hazardous | yes |
| Substances classified as hazardous and present in a mixture above the concentration limit and placed on the market after 1st of December 2010. (Those components of a hazardous substances which were not notified in REACH registration) | obligatory after 1 month of placed on the market | manufacturer, importer | – | Any quantity | hazardous | yes |
| After 1st of December 2010 first time manufactured or imported substances, which requires REACH registration | obligatory after 1 month of placed on the market | manufacturer, importer | Only-representative since the importer will do it | Above 1 tonnes | hazardous and non-hazardous | yes |
Notification tools
- IUCLID 5.2.2 & REACH-IT
- Bulk notification with an XML file & REACH-IT
- REACH-IT on-line notification
- Group of manufacturers and importers notification in REACH-IT
BIOCID
What shall I do if I manufacture an active substance in the EU?
The most important rule for you:
As of 1 September 2015, a biocidal product shall not be made available on the market if the manufacturer or importer of the active substance(s) contained in the product, or where relevant, the importer of the biocidal product, is not included in this list of ECHA:
List of active substance suppliers:
(ECHA updates this list regularly)
The first task is:
Since you manufacture biocide active substance, that is why your company should be on the list of active substance suppliers by 1st of September 2015.
If you are not on the list, as of 1 September 2015, you cannot manufacture the active substance, and none of your customers can make available on the market a biocidal product containing your active substance in EU.
If you want to be on the list of active substance supplier you should do the following tasks:
- To submit an inquiry dossier through R4BP system to get the contact of those companies, who has tests, and who has letter of access (LoA). If you are able to get the contact without inquiry dossier, than it is a faster solution.
- Ask the price of the Letter of Access (LoA) or
- You do/collect the tests alone according to Annex II and III. It is a huge task, without a good biocide consultant it will be hard to complete.
- You submit an active substance approval dossier
Active substance approval
The manufacturer or the importer of an active substance should submit to ECHA an application for approval of an active substance based on Article 6 through IUCLID and R4BP system by 1 September 2015, in order to be on the list of active substance suppliers.
It contains:
- a dossier for the active substance (Annex II.) or
- a Letter of Access (LOA) for the active substance and
- a dossier for the biocidal product that contain the acitve substance (Annex III.) or
- a Letter of Access (LOA) for the biocidal product that contain the acitve substance
Review program
The Commission will evaluate the active substance through a review program by the following deadlines:
| Priority | Existing active substances for product types |
All draft CARs have to be submitted to ECHA by |
The BPC have to submit all its opinions by |
| 1st priority list | 8, 14, 16, 18, 19, 21 | 31/12/2015 | 31/12/2016 |
| 2nd priority list | 3, 4, 5 | 31/12/2016 | 31/12/2017 |
| 3rd priority list | 1, 2 | 31/12/2018 | 31/12/2019 |
| 4th priority list | 6, 13 | 31/12/2019 | 31/12/2020 |
| 5th priority list | 7, 9, 10 | 31/12/2020 | 31/12/2021 |
| 6th priority list | 11, 12, 15, 17, 22, 23 (new PT20 under BPR) |
31/12/2022 | 31/06/2024 |
If the review of an approval of an active substance is successful, than the active substance can stay on the list of active substance suppliers for 5, 7 or 10 years.
What shall I do if I import an active substance from a non-EU country?
The most important rule for you:
As of 1 September 2015, a biocidal product shall not be made available on the market if the manufacturer or importer of the active substance(s) contained in the product, or where relevant, the importer of the biocidal product, is not included in this list of ECHA:
List of active substance suppliers »
(ECHA updates this list regularly)
The first task is:
Since you import a biocide active substance, that is why your company should be on the list of active substance suppliers by 1st of September 2015.
If you are not on the list, as of 1 September 2015, you cannot import the active substance, and none of your customers can make available on the market a biocidal product containing your active substance in EU.
If you want to be on the list of active substance supplier you should do the following tasks:
- To submit an inquiry dossier through R4BP system to get the contact of those companies, who has tests, and who has letter of access (LoA). If you are able to get the contact without inquiry dossier, than it is a faster solution.
- Ask the price of the Letter of Access (LoA) or
- You do/collect the tests alone according to Annex II and III. It is a huge task, without a good biocide consultant it will be hard to complete.
- You submit an active substance approval dossier
Active substance approva
The manufacturer or the importer of an active substance should submit to ECHA an application for approval of an active substance based on Article 6 through IUCLID and R4BP system by 1 September 2015, in order to be on the list of active substance suppliers.
It contains:
- a dossier for the active substance (Annex II.) or
- a Letter of Access (LOA) for the active substance and
- a dossier for the biocidal product that contain the acitve substance (Annex III.) or
- a Letter of Access (LOA) for the biocidal product that contain the acitve substance
Review program
The Commission will evaluate the active substance through a review program by the following deadlines:
| Priority | Existing active substances for product types |
All draft CARs have to be submitted to ECHA by |
The BPC have to submit all its opinions by |
| 1st priority list | 8, 14, 16, 18, 19, 21 | 31/12/2015 | 31/12/2016 |
| 2nd priority list | 3, 4, 5 | 31/12/2016 | 31/12/2017 |
| 3rd priority list | 1, 2 | 31/12/2018 | 31/12/2019 |
| 4th priority list | 6, 13 | 31/12/2019 | 31/12/2020 |
| 5th priority list | 7, 9, 10 | 31/12/2020 | 31/12/2021 |
| 6th priority list | 11, 12, 15, 17, 22, 23 (new PT20 under BPR) |
31/12/2022 | 31/06/2024 |
If the review of an approval of an active substance is successful, than the active substance can stay on the list of active substance suppliers for 5, 7 or 10 years.
What shall I do if I import a biocidal product from a non-EU country?
The most important rule for you:
As of 1 September 2015, a biocidal product shall not be made available on the market if the manufacturer or importer of the active substance(s) contained in the product, or where relevant, the importer of the biocidal product, is not included in this list of ECHA:
List of active substance suppliers »
(ECHA updates this list regularly)
The first task is:
Since you import biocide active substance together with the biocidal product, that is why your company should be on the list of active substance suppliers by 1st of September 2015.
If you are not on the list, as of 1 September 2015, you cannot import the active substance and the biocidal product containing your active substance, and none of your customers can make available on the market a biocidal product containing your active substance.
If you want to be on the list of active substance supplier you should do the following tasks:
- To submit an inquiry dossier through R4BP system to get the contact of those companies, who has tests, and who has letter of access (LoA). If you are able to get the contact without inquiry dossier, than it is a faster solution.
- Ask the price of the Letter of Access (LoA) or
- You do/collect the tests alone according to Annex II and III. It is a huge task, without a good biocide consultant it will be hard to complete.
- You submit an active substance approval dossier
Active substance approval
The manufacturer or the importer of an active substance should submit to ECHA an application for approval of an active substance based on Article 6 through IUCLID and R4BP system by 1 September 2015, in order to be on the list of active substance suppliers.
It contains:
- a dossier for the active substance (Annex II.) or
- a Letter of Access (LOA) for the active substance and
- a dossier for the biocidal product that contain the acitve substance (Annex III.) or
- a Letter of Access (LOA) for the biocidal product that contain the acitve substance
The Commission will evaluate the active substance through a review program by the following deadlines:
| Priority | Existing active substances for product types |
All draft CARs have to be submitted to ECHA by |
The BPC have to submit all its opinions by |
| 1st priority list | 8, 14, 16, 18, 19, 21 | 31/12/2015 | 31/12/2016 |
| 2nd priority list | 3, 4, 5 | 31/12/2016 | 31/12/2017 |
| 3rd priority list | 1, 2 | 31/12/2018 | 31/12/2019 |
| 4th priority list | 6, 13 | 31/12/2019 | 31/12/2020 |
| 5th priority list | 7, 9, 10 | 31/12/2020 | 31/12/2021 |
| 6th priority list | 11, 12, 15, 17, 22, 23 (new PT20 under BPR) |
31/12/2022 | 31/06/2024 |
Biocidal product authorisation
If the review of an approval of an active substance is successful, than the active susbtance can stay on the list of active substance suppliers for 5, 7 or 10 years and in 3 years the manufacturer or the importer of a biocidal product should submit the biocidal product authorisation dossier.
So for product-type 1 the review will be finished in 2018, and in 3 years later by 2021 the producer of the biocidal product should submit the biocidal product authorisation.
The biocidal product authorisation should be submitted through IUCLID and R4BP program. According to Article 20 it contains the following:
- a dossier for the active substance (Annex II.) or
- a Letter of Access (LOA) for the active substance and
- a dossier for the biocidal product that contain the acitve substance (Annex III.) or ú
- a Letter of Access (LOA) for the biocidal product that contain the acitve substance
The application should be submitted by the applicant or by its representative.
The biocidal product authorisation can be issued for a single biocidal product or for a biocidal product family.
Type of biocidal product authorisation:
- Union authorisation (valid in all member states, but very expensive) or
- National authorisation (valid in 1 member state)
The national authorisation can be extended for more member states:
- Mutual recognition in sequence or ( Article 33)
- Mutual recognition in parallel (Article 34)
So if you get the biocide authorisation for example for Hungary, than anybody can buy from you this product in Hungary, and your distributor do not need to get an own authorisation.
What shall I do if I prepare a biocidal product in the EU?
The most important rule for you
As of 1 September 2015, a biocidal product shall not be made available on the market if the manufacturer or importer of the active substance(s) contained in the product, or where relevant, the importer of the biocidal product, is not included in this list of ECHA:
List of active substance suppliers »
(ECHA updates this list regularly)
The first task is:
You need to know if your active substance supplier would be on the list of active substance suppliers by 1st of September 2015.
If your active substance supplier is not on the list, as of 1 September 2015, you cannot make available on the market a biocidal product containing this active substance.
If the answer is yes, and your active substance supplier is on the list, as of 1 September 2015, you can make available on the market a biocidal product containing this active substance, but please check the biocidal product-type, since the active susbtance approval related to a product-type.
Active substance approval:
(this is not the task of the distributor, but it is good if you understand this processt)
The manufacturer or the importer of an active substance should submit to ECHA an application for approval of an active substance based on Article 6 through IUCLID and R4BP system by 1 September 2015, in order to be on the list of active substance suppliers.
After it, the Commission will evaluate the active substance through a review program by the following deadlines:
| Priority | Existing active substances for product types |
All draft CARs have to be submitted to ECHA by |
The BPC have to submit all its opinions by |
| 1st priority list | 8, 14, 16, 18, 19, 21 | 31/12/2015 | 31/12/2016 |
| 2nd priority list | 3, 4, 5 | 31/12/2016 | 31/12/2017 |
| 3rd priority list | 1, 2 | 31/12/2018 | 31/12/2019 |
| 4th priority list | 6, 13 | 31/12/2019 | 31/12/2020 |
| 5th priority list | 7, 9, 10 | 31/12/2020 | 31/12/2021 |
| 6th priority list | 11, 12, 15, 17, 22, 23 (new PT20 under BPR) |
31/12/2022 | 31/06/2024 |
Biocidal product authorisation
(It should be prepared by the biocidal product’s producer)
If the review of an approval of an active substance is successful, than the active susbtance can stay on the list of active substance suppliers for 5, 7 or 10 years and in 3 years the manufacturer or the importer of a biocidal product should submit the biocidal product authorisation dossier.
So for product-type 1 the review will be finished in 2018, and in 3 years later by 2021 the producer of the biocidal product should submit the biocidal product authorisation.
The biocidal product authorisation should be submitted through IUCLID and R4BP program. According to Article 20 it contains the following:
- a dossier for the active substance (Annex II.) or
- a Letter of Access (LOA) for the active substance and
- a dossier for the biocidal product that contain the acitve substance (Annex III.) or
- a Letter of Access (LOA) for the biocidal product that contain the acitve substance
The application should be submitted by the applicant or by its representative.
The biocidal product authorisation can be issued for a single biocidal product or for a biocidal product family.
Type of biocidal product authorisation:
- Union authorisation (valid in all member states, but very expensive) or
- National authorisation (valid in 1 member state)
The national authorisation can be extended for more member states:
- Mutual recognition in sequence or ( Article 33)
- Mutual recognition in parallel (Article 34)
What shall I do if I distribute a biocidal product of an EU supplier?
The most important rule for you:
As of 1 September 2015, a biocidal product shall not be made available on the market if the manufacturer or importer of the active substance(s) contained in the product, or where relevant, the importer of the biocidal product, is not included in this list of ECHA:
List of active substance suppliers »
(ECHA updates this list regularly)
The first task is:
You need to know if your active substance supplier would be on the list of active substance suppliers by 1st of September 2015.
If your active substance supplier is not on the list, as of 1 September 2015, you cannot make available on the market a biocidal product containing this active substance.
If the answer is yes, and your active substance supplier is on the list, as of 1 September 2015, you can make available on the market a biocidal product containing this active substance, but please check the biocidal product-type, since the active susbtance approval related to a product-type.
This is not the task of the distributor, but it is good if you understand this process:
Active substance approval
The manufacturer or the importer of an active substance should submit to ECHA an application for approval of an active substance based on Article 6 through IUCLID and R4BP system by 1 September 2015, in order to be on the list of active substance suppliers.
After it, the Commission will evaluate the active substance through a review program by the following deadlines:
| Priority | Existing active substances for product types |
All draft CARs have to be submitted to ECHA by |
The BPC have to submit all its opinions by |
| 1st priority list | 8, 14, 16, 18, 19, 21 | 31/12/2015 | 31/12/2016 |
| 2nd priority list | 3, 4, 5 | 31/12/2016 | 31/12/2017 |
| 3rd priority list | 1, 2 | 31/12/2018 | 31/12/2019 |
| 4th priority list | 6, 13 | 31/12/2019 | 31/12/2020 |
| 5th priority list | 7, 9, 10 | 31/12/2020 | 31/12/2021 |
| 6th priority list | 11, 12, 15, 17, 22, 23 (new PT20 under BPR) |
31/12/2022 | 31/06/2024 |
Biocidal product authorisation
If the review of an approval of an active substance is successful, than the active susbtance can stay on the list of active substance suppliers for 5, 7 or 10 years and in 3 years the manufacturer or the importer of a biocidal product should submit the biocidal product authorisation dossier.
So for product-type 1 the review will be finished in 2018, and in 3 years later by 2021 the producer of the biocidal product should submit the biocidal product authorisation.
The biocidal product authorisation should be submitted through IUCLID and R4BP program. According to Article 20 it contains the following:
- a dossier for the active substance (Annex II.) or
- a Letter of Access (LOA) for the active substance and
- a dossier for the biocidal product that contain the acitve substance (Annex III.) or
- a Letter of Access (LOA) for the biocidal product that contain the acitve substance
The application should be submitted by the applicant or by its representative.
The biocidal product authorisation can be issued for a single biocidal product or for a biocidal product family.
Type of biocidal product authorisation:
- Union authorisation (valid in all member states, but very expensive) or
- National authorisation (valid in 1 member state)
The national authorisation can be extended for more member states:
- Mutual recognition in sequence or ( Article 33)
- Mutual recognition in parallel (Article 34)








